-
Characteristics of nuclear radiations:
|
Alpha (α) |
Beta (β) |
Gamma (γ) |
|
|---|---|---|---|
|
Nature |
Helium nucleus |
High speed electron |
Electromagnetic wave |
|
Ionizing effect |
High |
Medium |
Low |
|
Penetration |
Low (may be blocked by |
Medium (may be blocked by |
High (may be blocked by |
|
Deflection in magnetic field |
Yes (use Left-hand-rule) |
Yes (use Left-hand-rule) |
No |
|
Deflection in electric field |
Yes |
Yes |
No |
-
Radioactive decay: It is the break down of unstable nuclei in order to become more stable. In the process α, β or γ radiations are emitted.
-
Types:
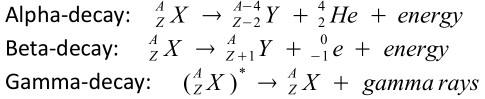
-
Asterisk (*) indicates that the nucleus is excited. Gamma rays are usually emitted at the same moment as either an alpha or beta particle.
-
-
-
Radioactivity is a phenomenon that is random in terms of:
-
time (You can’t exactly predict when a nucleus will decay.)
-
space (You can’t predict which nucleus will decay next.)
-
direction of emission
-
-
Methods of detecting radioactivity
-
Photographic plates are fogged.
-
Charged electroscope is neutralized because some ions made in air are attracted to it.
-
Diffusion cloud chamber shows tracks formed by alpha, beta or gamma radiations because alcohol vapour condenses on the ions formed.
-
GM-tube connected to a ratemeter or scaler.
-
Construction: See Fig 25.8 on page 398.
-
Working: When radiation ionizes argon gas inside GM-tube, an electrical pulse is produced.
-
-
-
Background radiation: It is the radiation in our surroundings due to:
-
cosmic rays from stars
-
underground radioactive rocks
-
-
Half-life: It is the time taken for half of the unstable nuclei to decay.
-
Uses of radioactive materials
-
tracers
-
penetrating radiation for thickness control or to reveal faults in weldings
-
nuclear fuel (e.g., Uranium-235)
-
treating cancer (by using gamma radiations)
-
archaeological dating (also known as carbon dating)
-
-
Hazards of radiations: Overexposure may lead to radiation burns, eye-cataracts, leukaemia (blood cancer), genetic mutations or even death.
-
Precautions
-
Keep distance by using forceps
-
Wear lead lined suits
-
Avoid eating and drinking
-
-
Conclusions from Geiger Marsden experiment (alpha scattering by gold foil):
-
Most alpha particles passed straight through. Therefore, most of the space in an atom is empty.
-
Few alpha particles were deflected by huge angles. Therefore, there must be a place (nucleus) where positive charges (protons) are concentrated.
-
-
Nucleon number (mass number) (A): It is the sum of the number of protons and the number of neutrons in an atomic nucleus.
-
Proton number (atomic number) (Z): It is the number of protons in an atomic nucleus.
-
Isotopes: Isotopes of an element are atoms which have the same proton number but different nucleon numbers.
-
Energy and mass can be inter-converted according to the formula:
E = m c²
where c is the speed of light in vacuum.
-
Nuclear fission: It is the process in which heavy unstable nuclides break up to produce energy.
-
Some related equations:
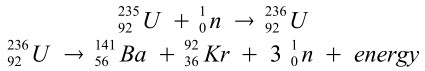
Note: If the 3 neutrons are made to collide with other Uranium-235 atoms, a chain-reaction starts.
-
-
Nuclear fusion: It is the process in which lighter nuclides fuse together to form a heavier nucleus with the release of energy.
- Star formation: Theories SUGGEST that a star is born within a giant cloud of dust (called nebula).

-
Turbulence deep within these clouds gives rise to knots with sufficient mass that the gas and dust can begin to collapse under its own gravitational attraction.
-
Friction raises temperature.
-
When the temperature is high enough, nuclear fusion of hydrogen starts (to form Helium) and a star is born.
Go back to table of contents



